Memphasys is a reproductive biotechnology company developing solutions for market needs in human and animal fertility and reproduction.
better technology, more life...
The Felix™ device for sperm separation
The Felix™ device is an automated electrophoretic system. The Felix™ device separates sperm from raw semen using a proprietary process which combines electrophoresis and size exclusion membranes. Sperm are harvested with exclusion of cellular contaminants such as leukocytes and precursor germ cells.
The Felix™ device consists of two main components: a console, which supplies electrical power, and a sterile disposable cartridge for sperm isolation and selection.
The Felix™ device is the first commercial product to be developed by Memphasys. The Felix™ device is currently being sold in international markets whilst clinical study and/or regulatory processes are underway.
Commercial status
ISO 13485
Memphasys has ISO 13485 certification of its quality management system. This accreditation means that the processes which are used to design, manufacture and market a technology such as the Felix™ device comply with the international ISO 13485 standard.
The Felix™ device also meets IEC 60601 standard and has passed MEA (mouse embryo assay) and HSSA (human sperm survival assay).
The Felix™ device is manufactured in a cleanroom environment and is available for sale in some early access markets (India, Canada, and NZ). Clinical study and/or regulatory processes are underway for other markets.
More information
RoXsta - (rapid in vitro antioxidant assessment)
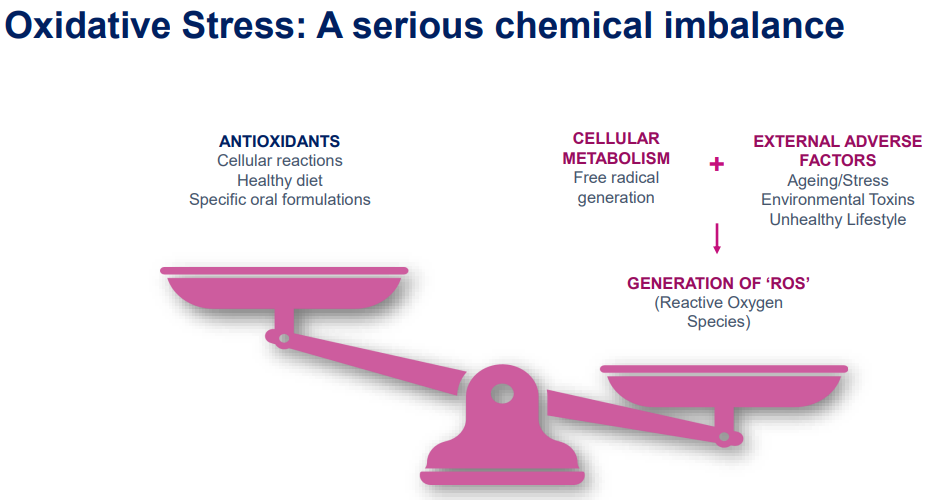
Oxidative stress is an imbalance between reactive oxygen species and antioxidant protection within the body. This imbalance tends to increase with age and can contribute to serious diseases. Oxidative stress can also severely affect fertility in both humans and animals1.
At present, there is no simple clinical assessment for oxidative stress. Rather, complex laboratory tests and analyses are required and therefore, not often used. Memphasys is developing a point-of-care diagnostic device. This diagnostic device is being investigated for application for bodily fluids (i.e., semen, blood and follicular fluid) for use in the IVF clinic and physician rooms.
The oxidative stress assay project will utilise a biochemistry-based assay. Subject to successful optimisation and validation, the prototype device will be field tested to demonstrate efficacy and determine market opportunities.
1. Aitken, RJ. (2022) The Infertility Trap. Cambridge University Press